Abstract
Introduction
High-dose chemotherapy with autologous stem cell transplantation (HDCT/ASCT) is a potentially curative treatment for men with metastatic germ cell tumors (GCT) in relapse after standard cisplatin-based chemotherapy. It is usually performed as tandem or triple transplantations and results in 2-year progression-free and overall survival rates of ~60%. However, a tandem ASCT is not always possible and outcomes for men treated with a single ASCT are poorly described. We reviewed our institutional experience of the use of HDCT/ASCT for men with relapsed GCT, aiming to ascertain reasons behind why a tandem transplant could not be completed, and the impact of a single ASCT on clinical outcomes and toxicity.
Methods
All men with metastatic GCT who underwent HDCT/ASCT at our institution between January 2000 and December 2016 were included in the study. After obtaining IRB approval, clinical data was abstracted from the medial record. Peripheral blood stem cells (PBSCs) were mobilized with G-CSF, with or without plerixafor; bone marrow harvests were reserved for men with failure to mobilize PBSCs. Conditioning was performed with carboplatin 700mg/m2 and etoposide 700mg/m2 (CE) or carboplatin 600mg/m2, etoposide 600mg/m2 and cyclophosphamide 50mg/kg/day (CEC) on days -5, -4 and -3; the regime chosen was at the discretion of the transplant physician. Progression-free (PFS) and overall survival (OS) were calculated as the duration from the first day of HDCT to date of relapse or death, or censored at last follow-up.
Results
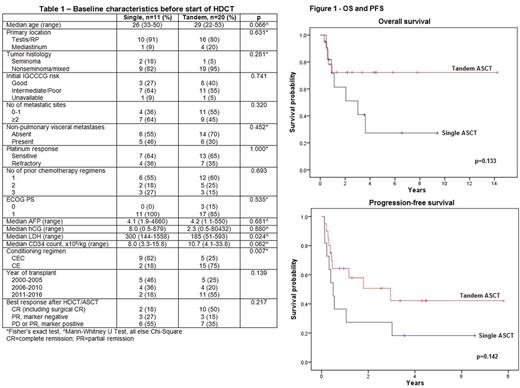
31 men underwent HDCT/ASCT for metastatic GCT between 2000-2016; median age was 30yrs (range 22-53) and median follow-up was 3.5yrs (95% CI 1.8-5.3). Clinical characteristics of patients before the start of HDCT, stratified by single (n=11) or tandem ASCT (n=20), are shown in Table 1. The two cohorts were well-matched with the exception that median LDH prior to HDCT was higher amongst patients who had a single ASCT (300 vs. 185, p=0.024), while a greater proportion of men who underwent a single ASCT were conditioned with CEC (82% vs. 25%, p=0.007).
(i) Single ASCT cohort
In the 11 men in whom a single ASCT was performed, a tandem had been originally planned in 8 (73%). 3 men, in the pre-plerixafor era, were unable to mobilize sufficient stem cells to permit a tandem ASCT, with 2 of these having to undergo a bone marrow harvest due to insufficient peripheral CD34 counts to permit apheresis (both had previously received 2 lines of platinum-based chemotherapy); the third patient had severe pain with G-CSF and was only able to collect sufficient cells (3.3x106/kg) for a single ASCT. Other reasons why men were unable to proceed to the second ASCT included disease progression with rising tumor markers after the first ASCT (n=3), poor performance status (n=1), and lack of insurance approval (n=1).
(ii) Toxicity
Any Grade 3 or higher non-hematologic toxicity occurred in all but one patient overall (n=30, 97%). Toxicity was similar between single and tandem ASCT, and did not lead to significant delays in proceeding with a tandem ASCT. Febrile neutropenia was very common, occurring in 82% and 85% of men who had a single or tandem ASCT respectively. There was notable ototoxicity, afflicting 27% of single and 25% of tandem ASCTs. There was no treatment-related mortality or leukemia.
(iii) Outcomes
A total of 12 men (39%) have died, all from progressive disease, 7 of whom had a single and 5 of whom had a tandem ASCT. Figure 1 shows PFS and OS stratified by single or tandem ASCT. Differences in median OS [3.0yrs (0.2-5.9) vs. not reached, p=0.133] or PFS [0.5 (0.3-0.7) vs. 3.0 (0.1-5.8), p=0.142] were not significant between single or tandem ASCT; 2-year PFS was 27% and 49%, and 2-year OS was 61% and 72% respectively. 10 of the 20 men (50%) who had a tandem ASCT are alive and disease-free after a median of 3.0yrs compared to 2 men (18%) who had a single ASCT.
Conclusions
A significant proportion of men undergoing HDCT/ASCT for metastatic GCT were unable to complete a planned second ASCT due to poor mobilization of PBSCs (mostly in the pre-plerixafor era) or rapid disease progression after the first ASCT. Toxicity was similar for men undergoing single or tandem ASCT and had minimal impact on ability to complete a second ASCT. There was a trend towards inferior survival among those who were unable to complete the second of planned tandem ASCT.
Ansell: Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Celldex: Research Funding; Seattle Genetics: Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal